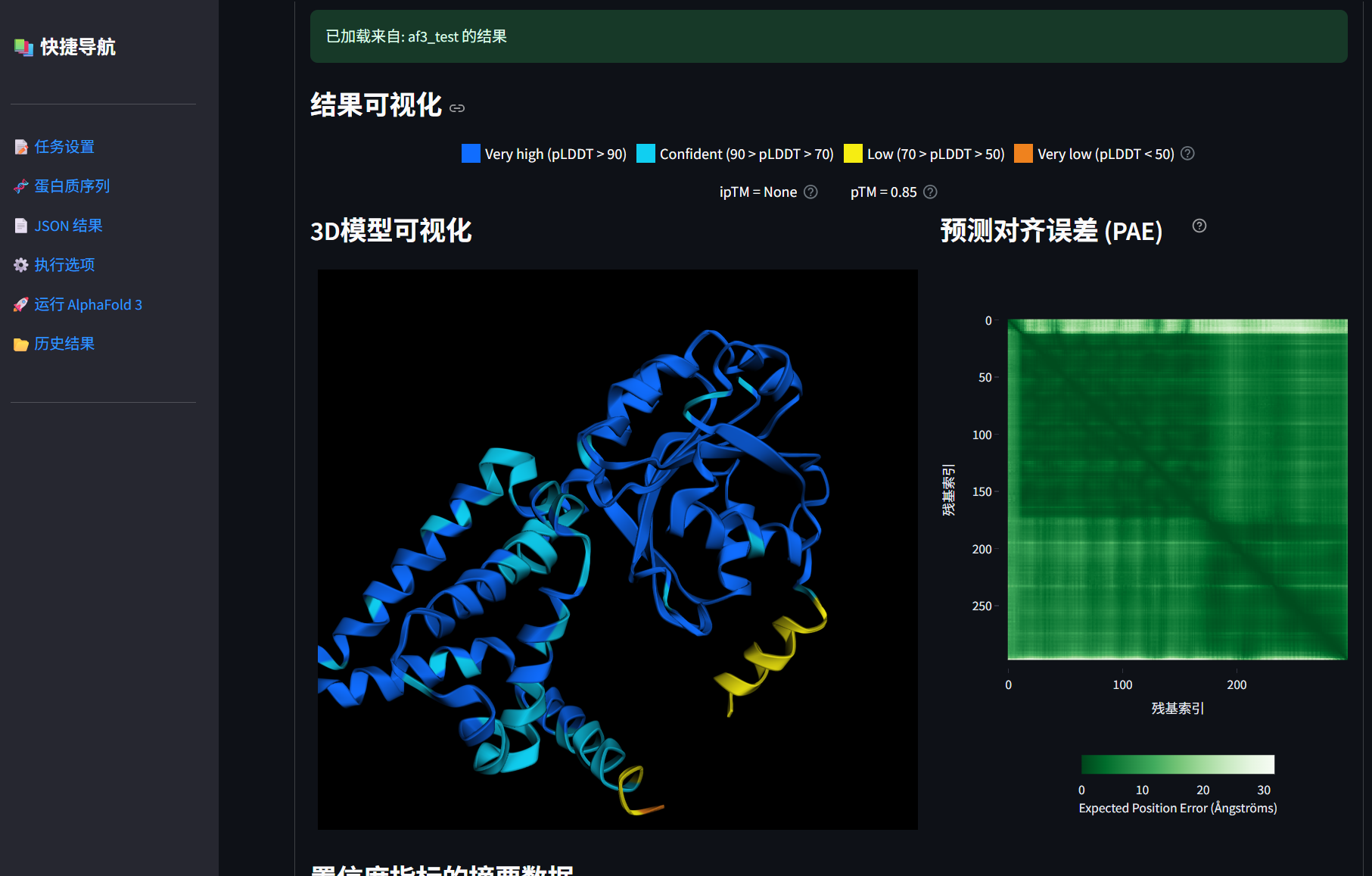
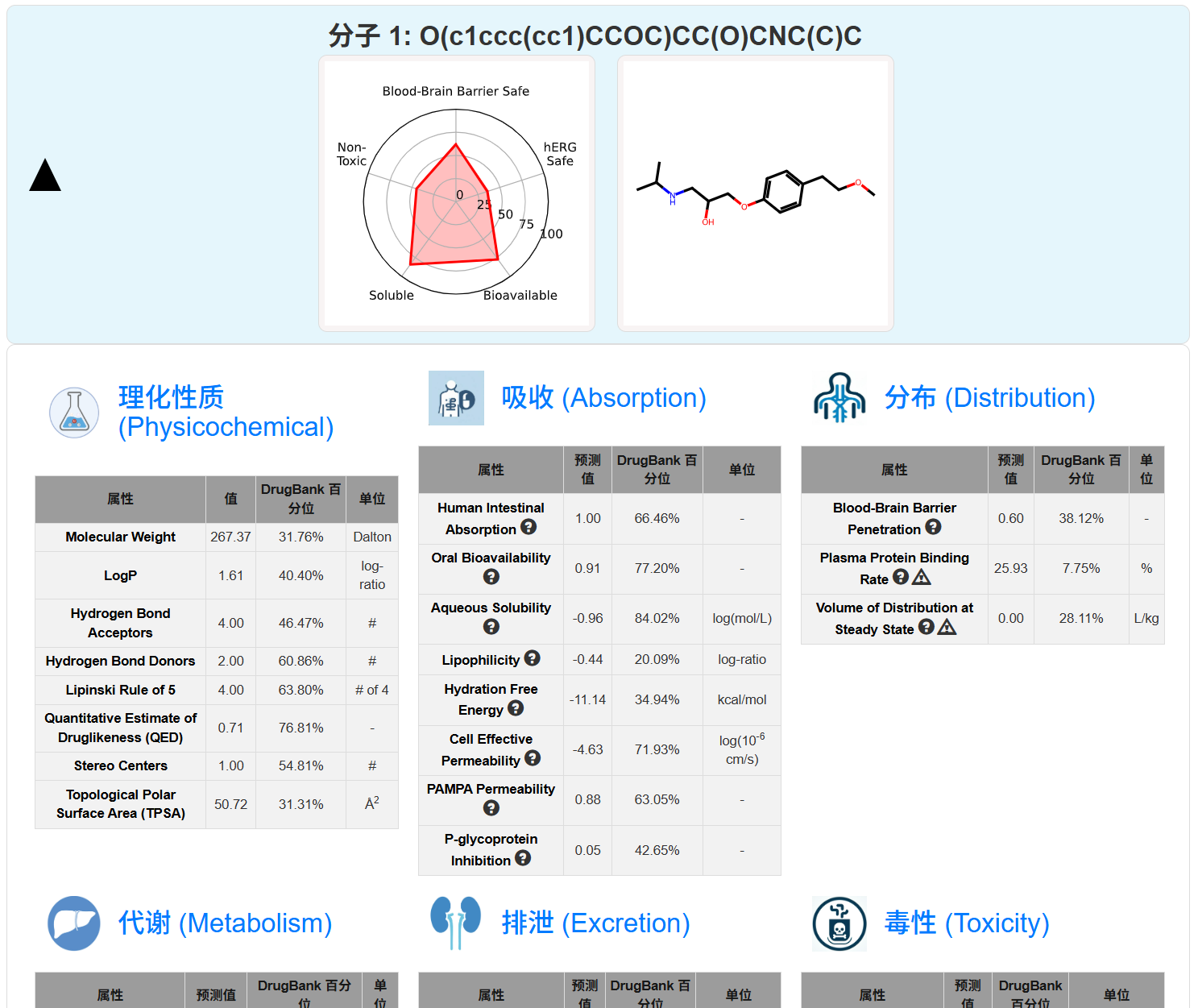
平台功能
算法 & 模型
数据库
论文
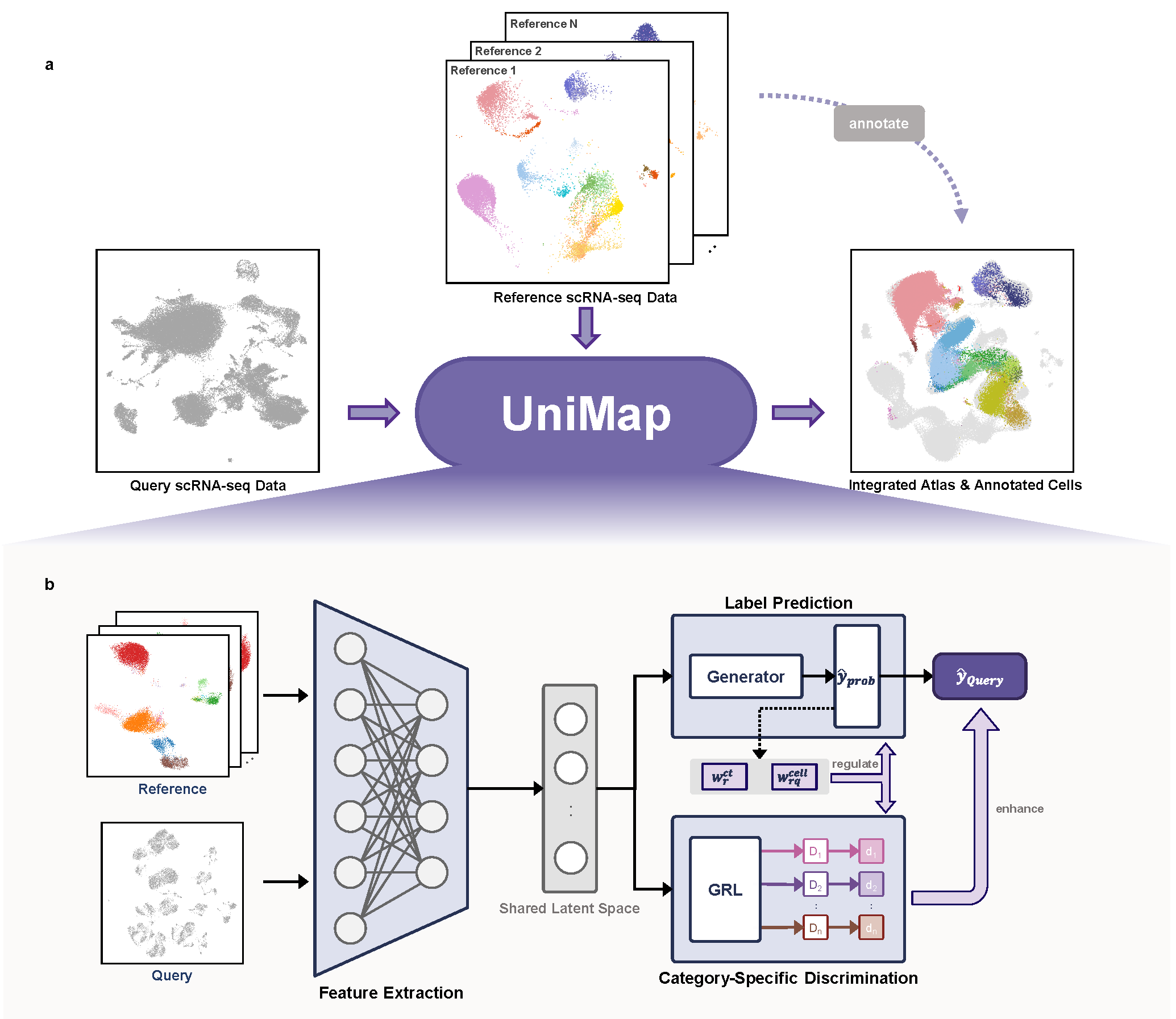
UniMap: Type-Level Integration Enhances Biological Preservation and Interpretability in Single-Cell Annotation
作者:Haitao Hu, Yue Guo, Fujing Ge, Hao Yin, Hao Zhang, Zhesheng Zhou, Fangjie Yan, Qing Ye, Jialu Wu, Ji Cao, Chang‐Yu Hsieh, Bo Yang
出版日期:2025年2月27日
期刊: Advanced Science
出版日期:2025年2月27日
期刊: Advanced Science
摘要: Integrating single-cell datasets from multiple studies provides a cost-effectiveway to build comprehensive cell atlases, granting deeper insights into cellularcharacteristics across diverse biological systems. However, current dataintegration methods struggle with interference in partially overlappingdatasets and varying annotation granularities. Here, a multiselectiveadversarial network is introduced for the first time and present UniMap,which functions as a “discerner” to identify and exclude interfering cells fromvarious data sources during dataset integration. Compared to other...
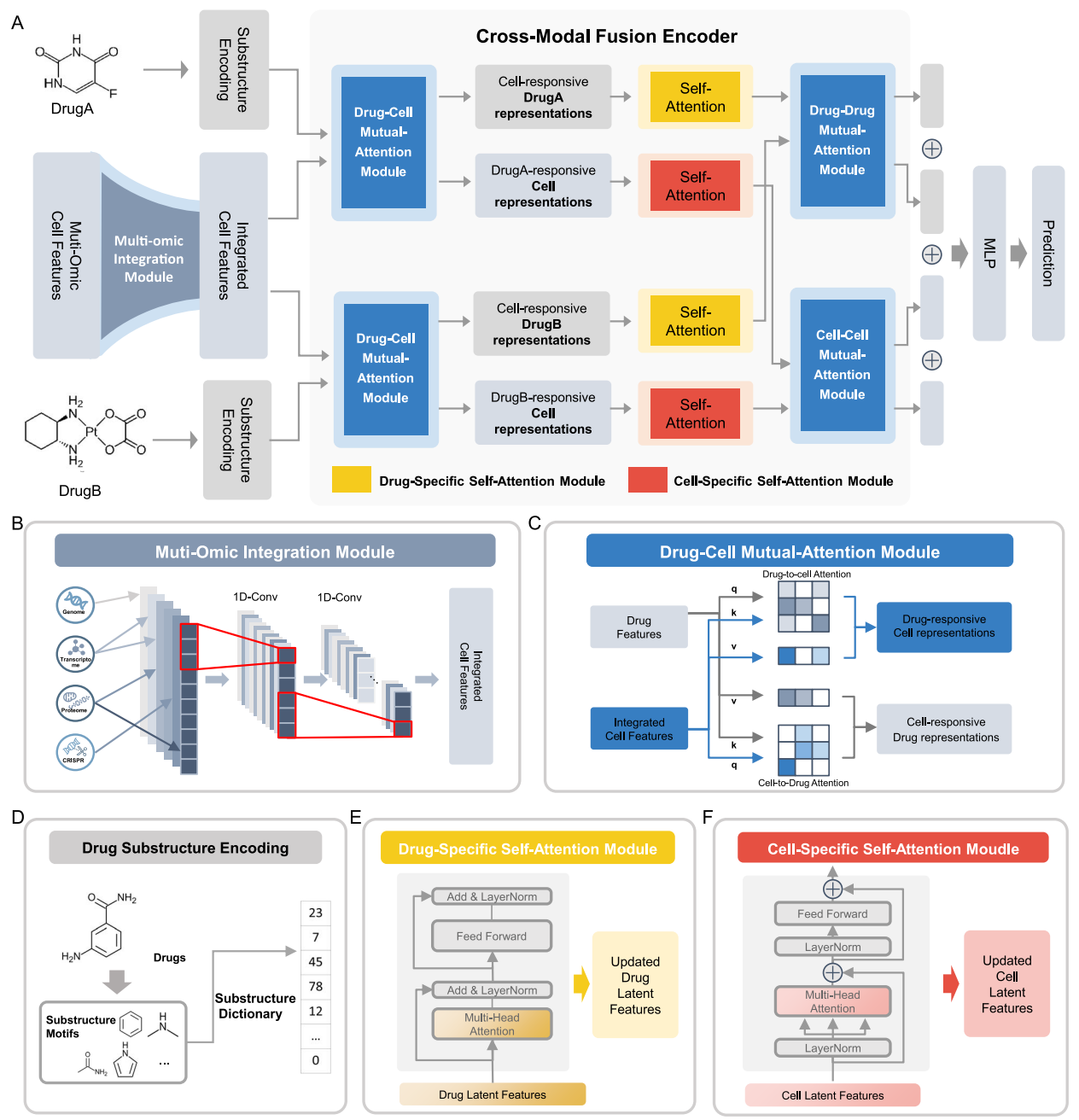
SynergyX: a multi-modality mutual attention network for interpretable drug synergy prediction
作者:Yue Guo, Haitao Hu, Wenbo Chen, Hao Yin, Jian Wu, Chang-Yu Hsieh, Qiaojun He, Ji Cao
出版日期:2024年2月10日
期刊: Briefings in Bioinformatics
出版日期:2024年2月10日
期刊: Briefings in Bioinformatics
摘要: Discovering effective anti-tumor drug combinations is crucial for advancing cancer therapy. Taking full account of intricate biological interactions is highly important in accurately predicting drug synergy. However, the extremely limited prior knowledge poses great challenges in developing current computational methods. To address this, we introduce SynergyX, a multi-modality mutual attention network to improve anti-tumor drug synergy prediction. It dynamically captures cross-modal interactions, allowing for the modeling of complex biological networks and drug interactions. A convolution-augmented attention structure is adopted to integrate multi-omic data in this framework effectively. Compared with other state-of-the-art models, SynergyX demonstrates superior predictive accuracy in both the General Test and Blind Test and cross-dataset validation. By exhaustively screening combinations of approved drugs, SynergyX reveals its ability to identify promising drug combination candidates for potential lung cancer treatment. Another notable advantage lies in its multidimensional interpretability. Taking Sorafenib and Vorinostat as an example, SynergyX serves as a powerful tool for uncovering drug-gene interactions and deciphering cell selectivity mechanisms. In summary, SynergyX provides an illuminating and interpretable framework, poised to catalyze the expedition of drug synergy discovery and deepen our comprehension of rational combination therapy.
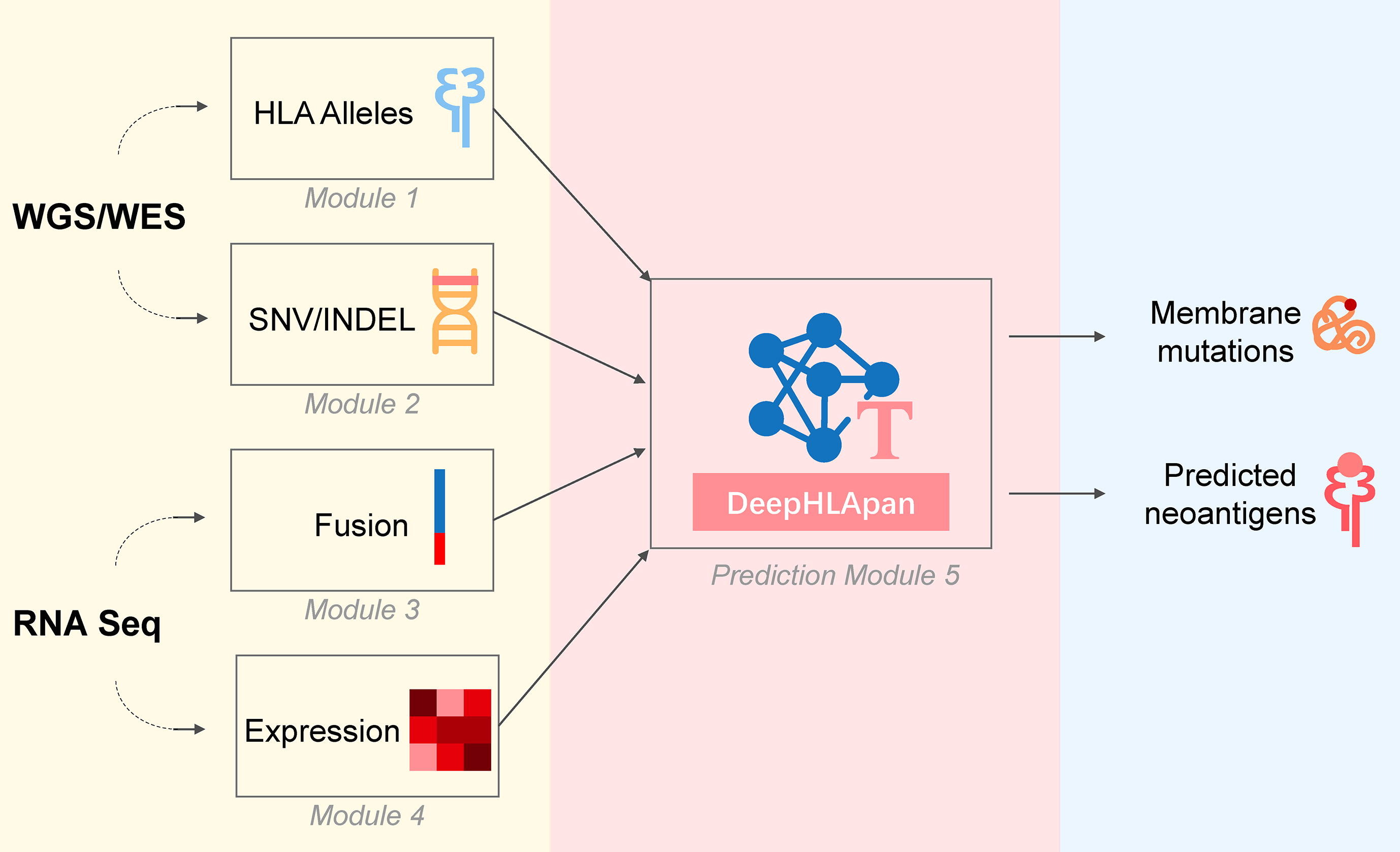
TSNAD v2.0: A one-stop software solution for tumor-specific neoantigen detection
作者:Zhan Zhou, Jingcheng Wu, Jianan Ren, Wenfan Chen, Wenyi Zhao, Xun Gu, Ying Chi, Qiaojun He, Bo Yang, Jian Wu, Shuqing Chen
出版日期:2021年8月12日
期刊: Computational And Structural Biotechnology Journal
出版日期:2021年8月12日
期刊: Computational And Structural Biotechnology Journal
摘要: TSNAD is a one-stop software solution for predicting neoantigens from the whole genome/exome sequencing data of tumor-normal pairs. Here we present TSNAD v2.0 which provides several new features such as the function of RNA-Seq analysis including gene expression and gene fusion analysis, the support of different versions of the reference genome. Most importantly, we replace the NetMHCpan with DeepHLApan we developed previously, which considers both the binding between peptide and major histocompatibility complex (MHC) and the immunogenicity of the presented peptide-MHC complex (pMHC). TSNAD v2.0 achieves good performamce on a standard dataset. For better usage, we provide the Docker version and the web service of TSNAD v2.0. The source code of TSNAD v2.0 is freely available at https://github.com/jiujiezz/tsnad. And the web service of TSNAD v2.0 is available at http://biopharm.zju.edu.cn/tsnad/.
承诺与愿景
专业性
我们的平台由浙江大学顶尖学者团队领衔打造,汇聚了包括国家杰青、长江学者在内的数十位AI与药学领域专家。团队成员不仅在国际顶级期刊发表多篇高影响力论文,更拥有领导国家级药物研发项目的丰富实战经验,确保了我们从底层算法到应用实践的绝对权威与前沿。
透明度
安全性
我们深知药物研发数据的极端重要性与机密性。平台依托国家级科研机构的严格管理体系与学术伦理标准,建立全方位的安全防护措施。我们承诺,所有用户数据与知识产权都将得到最高级别的保密与合规处理,为您探索下一代药物的宝贵工作保驾护航,绝不辜负您的信赖。